HEALTH
CDC Proposes Antibiotic ‘Morning-After Pill’ To Combat Rise in STDs
Published
9 months agoon
Courtesy of SFAF
The Centers for Disease Control and Prevention on Monday released a proposed plan to endorse the use of a common antibiotic by some groups of high-risk people as a morning-after pill to prevent certain sexually transmitted infections.
The proposed guidelines suggest that the intervention should only be offered to populations at increased risk for bacterial STIs like chlamydia, gonorrhea, and syphilis, in part because those are the most studied groups and in part to avoid overuse of the antibiotics, potentially leading to resistance.
The agency said cases of chlamydia, gonorrhea have been on the rise in recent years, with particularly jarring increases in syphilis and congenital syphilis.
The CDC proposed recommending the antibiotic protocol for gay men, bisexual men and transgender women who have had at least one bacterial STI in the last year, the groups considered to be most at risk.
The proposed guidelines are open to a 45-day public comment period.
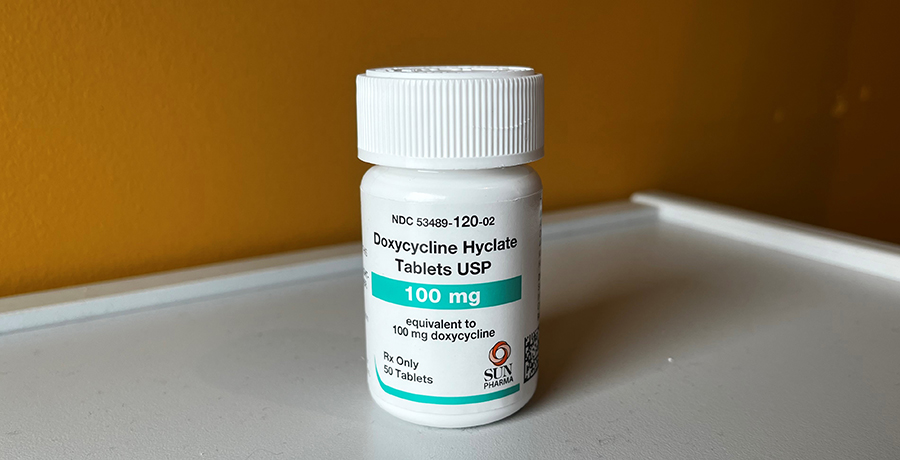
The CDC noted that recent studies have found that taking the antibiotic doxycycline within three days of unprotected sex significantly reduced participants’ likelihood of contracting chlamydia, syphilis or gonorrhea compared with people who did not take the antibiotic.
The agency said that although the use of doxycycline as a post-exposure prophylactic should be effective in other populations, there hasn’t been as much research on cisgender women, cisgender heterosexual men, transgender men, and others.
The San Francisco Department of Health already began recommending the use of doxycycline as a post-exposure prophylactic in October of last year, citing the need for solutions amid a dramatic increase in STIs.
Researchers at the agency, along with Zuckerberg San Francisco General, University of California, San Francisco, and the University of Washington, had recently conducted a controlled clinical trial on the drug protocol.
The agency began by recommending the protocol to cisgender men and transgender women who had at least one STI in the prior year and have sex with cisgender men or transgender women.
The agency said at the time that it was monitoring the anticipated results of a trial of the drug protocol in cisgender women in Kenya. There were concerns, however, for those using the drug after receptive vaginal sex, as doxycycline should be avoided during pregnancy.
TMX contributed to this article.
More From Sci + Tech
-


Netflix Launches Basic With Ads
-


XPENG AEROHT’s Flying Car Debuts in First Public Flight
-


Varda Capsule Earth Reentry Images. They Became the 3rd Private…
-


Deadly H5N1 Bird Flu Spreading To Marine Mammals After Adaptation,…
-


Love living and working so close to watch these launches!!!!
-


Satellite shows Hurricane Ian strengthening as it moves northward
-


More: Green Comet C/2022 E3 (ZTF) Seen from Ottawa, Canada
-


Japanese Billionaire Selects Civilian Crew For Private SpaceX Moon Mission
-


“Parachutes are deployed! The @SpaceX Dragon Freedom spacecraft with our…
-


Storm Babet lashes across western Europe as it brings heavy…
-


More: Researchers Add Pink Dye To Torrey Pines State Beach…
-


Jetson’s flying car completes world’s first EVTOL commute to work
