HEALTH
FDA Allows Florida To Import Bulk Prescription Drugs From Canada
Published
6 months agoon
Premier
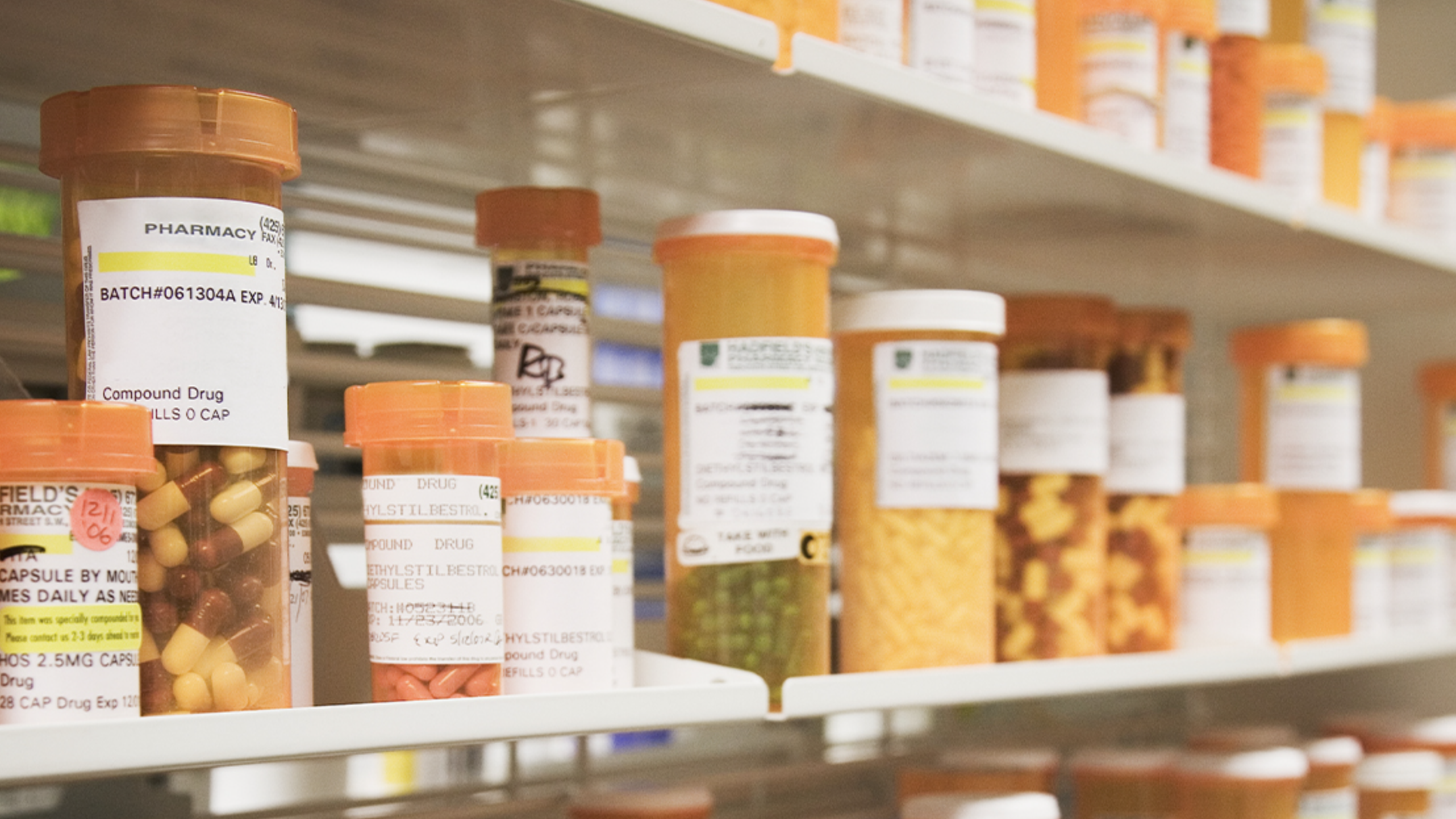
The U.S. Food and Drug Administration on Friday announced it will allow Florida to import prescription drugs in bulk from wholesalers in Canada under section 804 of the Federal Food, Drug, and Cosmetic Act.
President Joe Biden issued an executive order in 2021 directing the agency to work with states and tribal authorities on drug importation plans that reduce costs of consumers in the U.S.
Prescription drugs are frequently far less expensive outside the U.S., and multiple states, including Florida, have pushed the federal government to allow imports. The FDA already allows individuals to buy certain prescription drugs from Canada in some circumstances.
“The FDA is committed to working with states and Indian tribes that seek to develop successful section 804 importation proposals,” FDA Commissioner Robert M. Califf, M.D., said in a statement. “These proposals must demonstrate the programs would result in significant cost savings to consumers without adding risk of exposure to unsafe or ineffective drugs.”
The authorization is just the first step, however, as Floridas Agency for Health Care Administration must take additional steps, the FDA said.
The agency will have to submit drug-specific information to the FDA for review and approval; ensure the drugs it seeks to import have been tested for authenticity and FDA compliance; and relabel the drugs according to FDA-approved labeling.
Floridas Agency for Health Care Administration will also have to submit quarterly reports to the FDA detailing the drugs imported, cost savings and any potential safety and quality issues.
The authorization is valid for two years from the date the FDA is notified of the first shipment of drugs to be imported, the FDA said. The agency will maintain oversight of the program to ensure regulatory compliance.
TMX contributed to this article.
More From Sci + Tech
-


Robot Dogs ‘Sparkles’ and ‘Spot’ Meet, Do a Dance.
-

First Over-The-Counter Birth Control Pill To Hit US Stores This…
-


Colorful Auroras Seen From Oklahoma Mesonet Site During Solar Storm…
-


Frosted Cupcake Like Clouds on Jupiter – 3D Renders, from…
-


More: Meteor Seen Falling Through Sky from Wadsworth, OH
-


From Partial Eclipse on 10/14/23: Don’t have glasses to view…
-


Time-lapse of tonights Aurora from Nashville, Edgehill Neighborhood (5/10/24)
-


Meteor Seen Over Berlin on 1/21/24.
-


More: NASA Orion Capsule Captures the Dark Side of The…
-

Paddington Bear Lookalike Crater Found on Mars – The Image…
-


SpaceX Falcon 9 Launches Transporter-6 Rideshare Mission
-


More – Possible Meteor Seen From Belfast, Ireland
