HEALTH
FDA Approves First Gene Therapies For Sickle Cell Disease, With First US Approval For CRISPR Use
Published
7 months agoon
Dr. F. Gilbert/CDC
The Food and Drug Administration on Friday announced it has approved two groundbreaking gene therapies for the treatment of sickle cell disease, one of which is the first therapy utilizing the gene-editing tool CRISPR to be approved in the U.S.
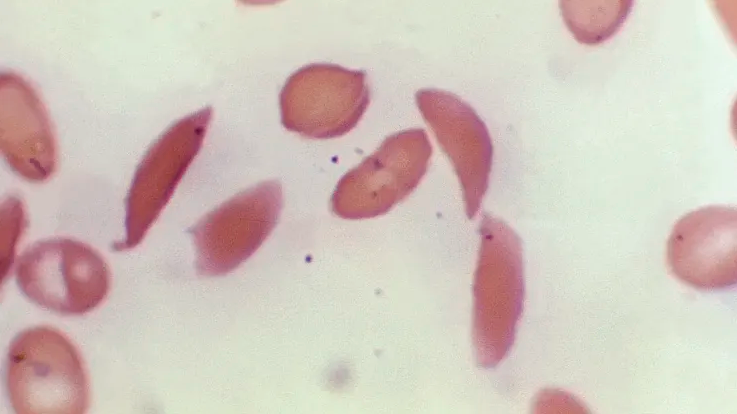
Sickle cell disease is a debilitating blood disorder affecting around 100,000 Americans, predominantly African Americans, though it also affects some Latinos. In sickle cell disease, blood cells can sickle, becoming misshapen, restricting blood flow and depriving organs and tissues of necessary oxygen, leading to severe pain and organ damage called vaso-occlusive events.
Previously, the only cure for the condition was a bone marrow transplant, which involved the difficulty of finding a matching donor and the risk of rejection by the patient’s immune system.
“Sickle cell disease is a rare, debilitating and life-threatening blood disorder with significant unmet need, and we are excited to advance the field especially for individuals whose lives have been severely disrupted by the disease by approving two cell-based gene therapies today,” Dr. Nicole Verdun, director of the Office of Therapeutic Products within the FDAs Center for Biologics Evaluation and Research, said in a statement. “Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited.”
Casgevy, from Vertex Pharmaceuticals and CRISPR Therapeutics, eliminates the need for marrow donation, by editing the DNA in the patient’s stem cells, removing the gene that causes the disease. those modified stem cells are then returned to the patient’s body where they attach and multiply within the bone marrow. It was approved for patients 12 and older.
It is the first treatment approved in the U.S. that utilizes CRISPR/Cas9 gene-editing technology, for which its inventors won the Nobel Prize in chemistry in 2020.
The second approved therapy is Lyfgenia, a cell-based gene therapy from drugmaker Bluebird Bio. Rather than using a gene-editing technology, it uses a lentiviral vector, or a gene delivery vehicle, for genetic modification. With Lyfgenia, the patients blood stem cells are genetically modified to produce a modified hemoglobin similar to hemoglobin A, which is the typical adult hemoglobin in adults without sickle cell disease. It was also approved for patients 12 and older.
Both treatments use the patients own blood stem cells, which are modified, and are given back as a one-time, single-dose infusion. Prior to treatment, a patient’s stem cells are collected, then the patient must undergo high-dose chemotherapy to remove cells from the bone marrow so they can be replaced with the modified cells.
“These approvals represent an important medical advance with the use of innovative cell-based gene therapies to target potentially devastating diseases and improve public health,” said Dr. Peter Marks, director of the FDAs Center for Biologics Evaluation and Research. “Todays actions follow rigorous evaluations of the scientific and clinical data needed to support approval, reflecting the FDAs commitment to facilitating development of safe and effective treatments for conditions with severe impacts on human health.”
In trials, patients treated with Casgevy remained free of vaso-occlusive events for at least 12 consecutive months within a 24-month followup period. The most common side effects were low levels of platelets and white blood cells, mouth sores, nausea, musculoskeletal pain, abdominal pain, vomiting, fever, headache and itching.
Among patients treated with Lyfgenia 88% saw complete resolution of vaso-occlusive events between six and 18 months after treatment. However, some patients developed blood cancer, and the treatment will come with a black box warning for cancer risk. The FDA said patients treated with Lyfgenia should have lifelong monitoring for possible cancer.
TMX contributed to this article.
More From Sci + Tech
-


2023 Solar Eclipse Casts Ring Of Fire Shadows Through Tree…
-


NASA – Christmas Tree Cluster, a swarm of stars and…
-


Kīlauea Volcano – Sizable bright orange lava fountain and fast…
-


Supermoon Appears In Seattle, Washington
-


Goodland, KS: Small swarm of meteors visible low on the…
-

CIRA Satellites Show Image of Smokehouse Creek Fire on Texas/Oklahoma…
-

CIRA Satellites: Fog and low-level clouds permeated across parts of…
-


Poteet, TX, Ring of Fire Eclipse captured just outside of…
-


NASA astronomers witness for the 1st time a dead star…
-


CIRA Eclipse Imagery, The complete, incredible view of the Great…
-


Colorful Auroras Seen From Oklahoma Mesonet Site During Solar Storm…
-

Tiny Marine Worm Has Enormous Eyes That See As Well…
