HEALTH
FDA Warns Counterfeit Botox Linked To Outbreak In Multiple States
Published
2 months agoon

More From Sci + Tech
-


Amazon Announces New Project Kuiper Satellite Production Facility
-


Seattle, WA – Timelapse of crescent moon moving over the…
-


NASA astronomers witness for the 1st time a dead star…
-


NASA Earth Observatory Releases Satellite Images of Dubai, Abu Dhabi…
-


Electric-Car Maker Polestar Is Branching Out With A New, Sustainable…
-


NASA reveals stunning images from International Space Station
-


NASA, Lockheed Martin Unveil X-59, A Quiet Supersonic Plane With…
-
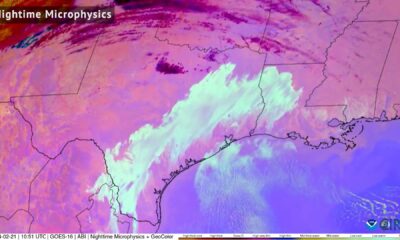

CIRA Satellites: Fog and low-level clouds permeated across parts of…
-


Apple Stock Falls As Protests In China Threaten iPhone Production
-


Tiny robotic crab is smallest-ever remote-controlled walking robot
-


Satellites Show Tropical Storm Franklin Making Landfall in Barahona, Dominican…
-


SkyFi’s Satellite Images Show Massive Clothes Pile Dumped in Chile’s…
