HEALTH
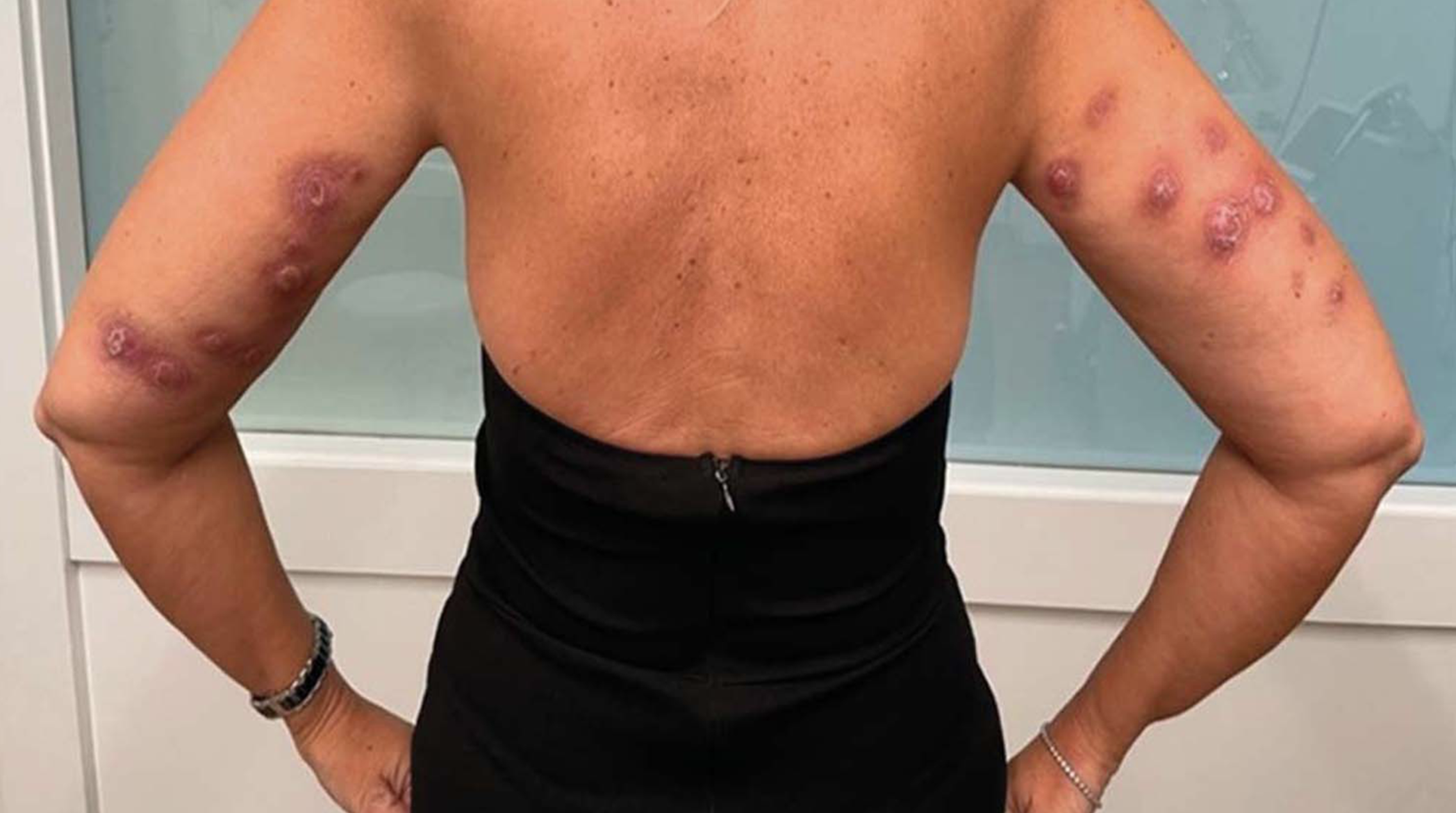
FDA Warns Of Serious Side Effects From ‘Fat-Dissolving’ Injections Not Approved By The Agency
Published
6 months agoon
More From Sci + Tech
-


More: SpaceX Falcon Heavy Launches Mission for Space Force
-
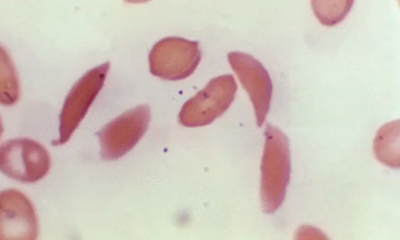
FDA Approves First Gene Therapies For Sickle Cell Disease, With…
-


Samsung Recalls 663,500 Washing Machines Over Fire Hazard
-


CIRA Satellites Show Low Pressure System Moving towards North America…
-


Seattle, WA – Timelapse of crescent moon moving over the…
-


Colorful Aurora from Solar Storm Seen from Spokane, WA (05/11/24)
-
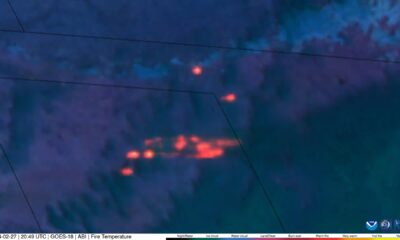

CIRA Satellites Show Image of Smokehouse Creek Fire on Texas/Oklahoma…
-


Colorful Auroras Seen From Oklahoma Mesonet Site During Solar Storm…
-

Sphere Entertainment Showcases Hi-Tech Atrium of Interactive Sphere Venue for…
-


Satellites Show Tropical Storm Franklin Making Landfall in Barahona, Dominican…
-


The eye of tropical cyclone #Freddy – captured live from…
-


Tropical Storm Bret Forms in Atlantic, Anticipated to Become A…
